Nicotine pouches are exciting products that can help support worldwide tobacco harm reduction....


2 minute read
The stability of cannabinoid products is a critical factor for manufacturers aiming to deliver high-quality, safe, and effective products. Cannabinoids, particularly tetrahydrocannabinol (THC) and cannabidiol (CBD), are naturally occurring compounds...
Filter
Filter

Nicotine pouches are exciting products that can help support worldwide tobacco harm reduction....
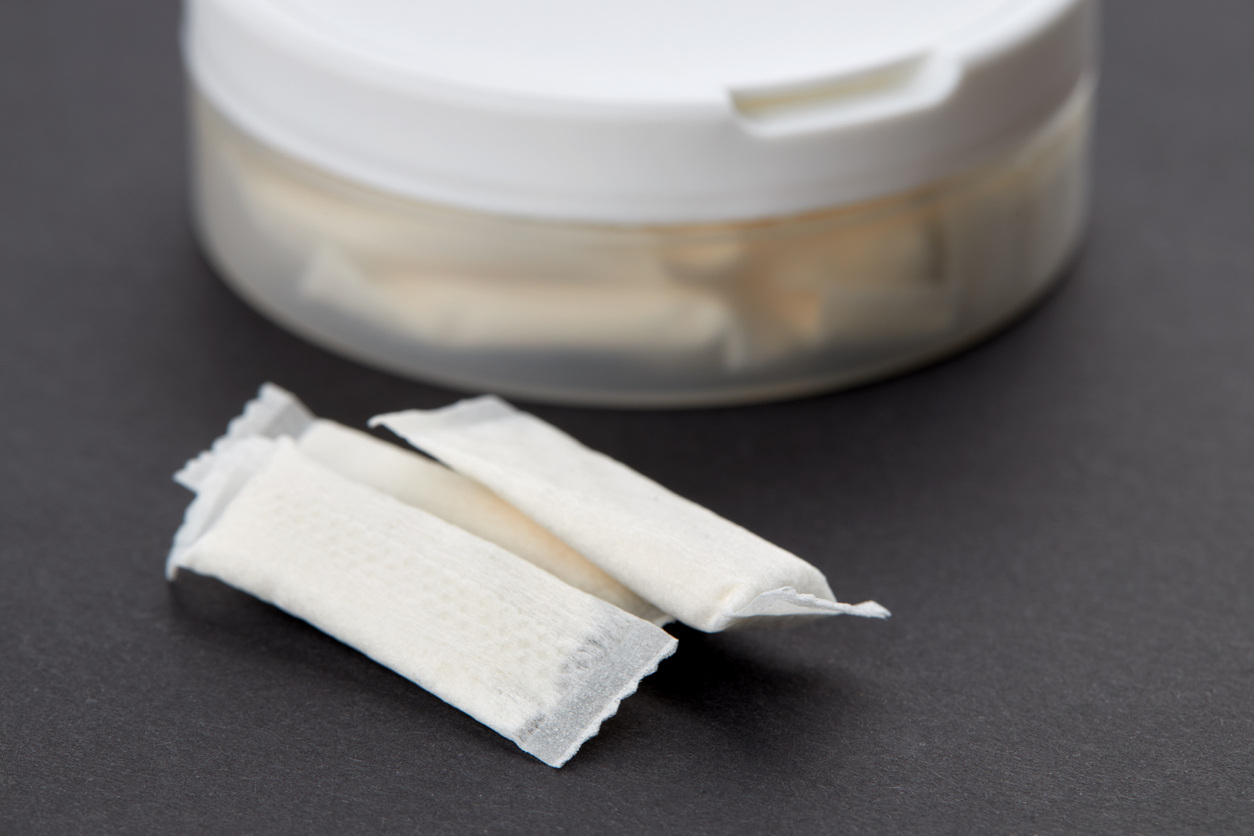
As the global tobacco and nicotine market evolves, nicotine pouches are emerging as a popular...

Broughton welcomes the US Food and Drug Administration’s (FDA) decision to authorize ZYN nicotine...

3 minute read
Navigating FDA and ICH Guidelines: Analytical Testing Validation for Tobacco Products
-2.jpg)
1 minute read
Oral nicotine pouches have become a disruptive force in the reduced-risk nicotine product...

Allergies concerns highlights need to meet stringent regulations ~

2 minute read
In the stringent and highly regulated pharmaceutical industry, reliable stability study results are...

2 minute read
When it comes to ensuring the safety, quality, and compliance of pharmaceutical products, stability...

2 minute read
The cannabis industry is on track for transformative growth in 2025, driven by advancements in...
Ensure your business stays ahead of the competition by keeping up to date on the latest regulatory changes. Sign up for our newsletter to have the latest information and insights served directly to your inbox.